
Ian Meng in his lab
http://itsa.ucsf.edu/~synapse
http://itsa.ucsf.edu/~synapse/archives/nov20.97/meng.html
UCSF synapse November 20, 1997
Volume 42, Number 12
Medical Marijuana: Doing the Science
In late October, postdoc Ian Meng presented a paper
at the Society for Neuroscience meeting in New Orleans, summarized thus in a UCSF press
release and newspapers across the world: "a synthetic marijuana-like drug called WIN
55212 enhances the brain's ability to suppress pain in rats, and probably in humans as
well."
This debriefing was conducted in early November, back in the crowded lab on
the 7th floor of Medical Sciences that Meng shares with co-investigators Barton Manning,
PhD, and Howard Fields, MD, professor of neurology, and nine others. Meng is 28; he
did his undergraduate work at Brown, and got his PhD there.

Ian Meng in his lab
Synapse: Who decided to name a
cannabinoid WIN 55212-2?
IM: The company that distributes it is called
RBI. [Research Biology International. The compound was synthesized by Sterling
Winthrop in 1990.] Right now there are three good synthetic cannabinoid agonists that act specifically on the CB-1 receptor. There's
been a big breakthrough in the last year, because they now have a specific antagonist, which lets you be sure that the compound is having its
effect on the specific receptor.
Synapse: In your study, who took the
initiative: you or RBI?
IM: I did. Actually, the antagonist is now free from National Institute of Drug Abuse
(NIDA). I just gave them a call and did the paperwork.
Synapse: Why is NIDA making the antagonist available? In case somebody accidentally ingests
some marijuana?
IM: Well, marijuana is not lethal, so
that's not a big concern. Recently it's been made available because they want more
studies done on cannabinoids. They're actually getting on the bandwagon and starting
to support this kind of research. It's been a big switch.
Until recently the government was only willing to fund research that
asked questions about the possible negative side effects of marijuana. But there
have been so many anecdotal reports of marijuana helping people with a variety of diseases
that people in California and Arizona voted to legalize marijuana for medical purposes.
The voters said, "We're going to use it anyway," so all of a sudden the
government is saying, "We'd better start funding research to really see what's
happening."
Synapse: That is news... Could you
define a cannabinoid?
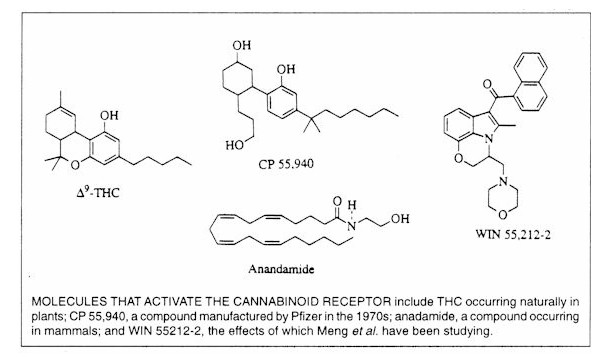
IM: The most active ingredient in cannabis
is the delta-9 THC molecule, which binds with a certain affinity to a specific receptor in
the brain known as the CB-1 receptor. There have now been two proposed endogenous ligands for this receptor. So cannabinoids are drugs that bind to
the CB-1 receptor. There are less active ingredients in marijuana which may have
some role in its effects. That's why we need to do studies where we compare the
effects of smoking marijuana to the effects of specific agonists.
You do want to know whether there are other components in the actual marijuana
influencing the effect.
Synapse: Have all the different cannabinoids
been isolated? Wouldn't you want to know what's in the plant first before you start
working with synthetics?
IM: I think most of that work was done in
the 1970s. For the kinds of studies I do I want to know that a particular drug is
acting at a specific receptor. That's why I use synthetic compounds... (Takes
a 20 ml vial of clear fluid from a desk drawer). It comes in a powdered form, I mix
it in an oil-based solution and give it intravenously, and I look at the activity of
single cells in this particular brain region which can modulate pain. That's the
basic paper I presented at the neuroscience meeting. I found that when I give the
cannabinoids intravenously, it will affect a group of cells in one of the brain's pain
areas. It's thought that these cells, when activated, reduce the amount of pain
allowed to be transmitted up through the spinal cord to the brain, where sensation occurs.
So when the cells in this area are activated, they inhibit pain. Morphine
activates these cells to inhibit pain; and so -- we found looking at single cells -- do
cannabinoids.
I wanted to show that the activity of these cells was related to the
analgesia being produced. So the second step was to give the drug to awake rats
systemically and then do a microinjection into this brain region to inhibit all cell
activity. Under those circumstances, the animals do not show an analgesic effect
after the systemic cannabinoid. The activity of cells in this brain region is
necessary for the cannabinoid to have its analgesic effect.
Synapse: So you're trying to figure out how
cannabinoids work -- you're not trying to prove that they do work. You recognize the
existing evidence.
IM: Yes. People know that they're
analgesic. But unitl fairly recently it hadn't been proven in animal studies that
cannabinoids affect sensation. When you do pain research it's important to
differentiate between the motor effects a drug might have versus the sensory effects.
If you give a drug that has a motor effect -- that decreases the rat's activity,
for example -- then some observers might say, "That explains the apparent decrease in
pain..." You can't ask an animal , "Do you feel less pain?" You
have to look at certain behaviors. What I do is called the tail flick. [Meng
has a testing device in which the heat source is a light bulb inside a gray metal box.
The anesthetized rat is placed on the box with its tail in a groove above a hole,
so that the radiant heat from the bulb will reach the tail, which the animal then flicks
out of the groove. More time before flick = feeling less pain.] If you give an
analgesic drug, a cannabinoid, or morphine, then the animal will leave its tail on the
heat source much longer. You can also do this tail-flick in another set-up through a
glass bottom with freely moving, unanesthetized rats.
I also look at the actual transmission of pain signals through the
central nervous system. I do single cell recording -- looking at the activity of
single neurons that are related to pain. I can actually look at the electrical
impulses that travel down neurons to tell me how active a cell is. By doing that,
we've been able to show, it's not just motor effects; this cannabinoid has very specific
sensory effects. It affects the neurons in the pain pathway.
Synapse: So where is this research heading?
What happens next?
IM: One thing I'm very interested in is,
why is there this endogenous cannabinoid system in the body? It's got to be there
for some reason. And it has to be activated under certain types of conditions.
It's been known for a long time that certain types of stress will activate the
endogenous opioid system so the endorphins and those kinds of things can kick in.
Say if somebody gets their arm blown off in a war, they won't feel any pain because this
pain-modulating brain center that I record in is activated, and it shuts off pain before
it can reach the part of the brain that coordinates sensation. And that's important
for survival because you don't want to be distracted by your pain, you want to get out of
there.
So certain kinds of stress involve opioids. The cannabinoid
system is a very separate system but it activates the same kind of neurons. One
hypothesis is that there are different kinds of stress that activate the cannabinoid
system.
Synapse: Why do plants contain cannabinoids?
And why do poppies contain opium? Do you find yourself pondering the big
evolutionary questions?
IM: And the bark of the willow tree for
aspirin... Yeah, there are a lot of natural substances which have specific actions
that reduce pain. I'm not sure why plants have evolved to make these compounds, but
it has really helped scientists gain insight into the way the brain works. It also
makes me believe that the Western scientific and medical community could learn something
from people in other parts of the world who use all kinds of herbal and other natural
remedies.
Synapse: Why did the company want to develop
a synthetic cannabinoid in the first place? Why not study the naturally occurring
ones first? Were they trying to create a patentable molecule? Or a legal
molecule?
IM: The real reason is, when you make a
synthetic compound it can actually be more potent and more effective.
Synapse: Why is that?
IM: It can bind to the receptor molecule
better. You can target that receptor. It can also have a longer duration of
action.
Synapse: Is that what the makers of WIN
55212-2 did?
IM: Actually, I think the WIN compound was
discovered by accident. They were looking for something completely different.
Synapse: They weren't tweaking naturally
occurring cannabinoids?
IM: Not at all.
Synapse: And this drug you're working with
is a cannabinoid because it binds to the cannabinoid receptor?
IM: Yes. And now they have other
synthetic compounds that have been screened for activity at the cannabinoid receptor.
Synapse: What makes a molecule want to bond
with the cannabinoid receptor? Is there some chemical group offering a special
handshake?
IM: Basically, it's got to do with the
makeup of the receptor. You have certain amino acids and certain positive and
negative charges which have to match both the shape and the charges on the drug. So
you can do computer models to try to figure out what would be a good synthetic compound.
But those usually aren't that good at predicting what's going to bind.
Normally, it's just making a lot of compounds and screening them [for function by biologic
assay] and seeing what works.
Synapse: Do you talk to people back at the
company? Are they following your work? Could your work translate into big
bucks for this company?
IM: I really don't know. I've come to
this purely through scientific interest and I never even think about that kind of thing.
I probably should.
Synapse: When I read the story out of New
Orleans, I thought about those chemists in Basel 30 years ago, tweaking the amphetamine
molecule to come up with Ritalin.
IM: No, we really rely on what the chemists
give us. They give us the tools, and then we can kind of figure out what's
happening...
Synapse: I have a vested interest in seeing
that the appropriate research is done on any drug that offers hope in the treatment of
epilepsy.
IM: Of course there have been anecdotal
reports of people using marijuana to help their epilepsy. I'm sure it's just a
matter of time before scientists start looking at that actual mechanisms by which
cannabinoids can control seizures. It's through this type of research that new and
better treatments could result.
Synapse: Has your life changed since your
paper was published?
IM: (laughs) A little bit. One
part is people calling up and wanting to know how they can get this drug to help them,
because there are a lot of people with really severe chronic pain for whom nothing to this
point has worked. So we've gotten some calls like that. Then you've got
reporters calling, wanting to know how it affects the whole political debate.
Synapse: And what's your line on that?
IM: My line is that it should be legal.
It definitely should be legal for people who need it to help with an illness or a
disease like chronic pain or epilepsy. And cannabis can really help. Basically
the science is just showing that there are very specific mechanisms by which cannabis can
help. People are taking this as a medicine, and for very specific reasons.
It's hard to get that point through.
Synapse: We've all had a lifetime of
prejudice and propaganda.
IM: Absolutely... It's satisfying to
really do the science.
An agonist is a drug which binds to and activates a receptor. An antagonist prevents the agonist from binding. A ligand is a compound, endogenous or synthetic, that binds to a receptor.
Q&A with Howard Fields
Howard L. Fields, MD, PhD is vice-chair and
professor, Neurology and professor, Physiology at UCSF.
Synapse: How could you have been in the field for 30 years
and not realized that marijuana had medical applications?
HF: It never occurred to me. I assumed that the drugs we
had were good enough but underutilized. Meaning morphine, codeine... The
opioid-containing medications tend to be underutilized. Patients -- and doctors --
are deathly afraid that they'll become addicted. I've know a lot of people who have
used marijuana, and I've used it myself in the past, and was never really aware that it
was analgesic. Now people are coming along and saying it seems to be very good as an
analgesic. If they're right, that's great.
To a layman it seems odd that when we haven't studied the naturally
occurring cannabinoids, we're studying a synthetic.
You can't study the naturally occurring ones very well.
Why not?
You don't know the concentration of the effective agent.
It's different from batch to batch. With the synthetics you know exactly how much
you're administering. And it's a simpler compound. Now you could say "Why
not take the natural product, see if you get analgesic effect and then block it with the
antagonist."
Yes, you could...
We now know two of the receptors for cannabis. And we know
that you've got a substance that's present in the natural product and acts at that
receptor. So if it gives you pain relief, you know that at least one agent in the
natural product does, and you have an idea about how it does it. That leaves two
problems. One: there might be something else in the natural product other than what
we know about and that what we know acts at the cannabinoid receptor that might add to its
effectiveness; or there might be something there that detracts from its effectiveness.
Or there might be things that produce side effects or are toxic. So in my
mind you're always better off with a known molecule when you're doing research.
What does it mean to say that a plant contains 400 cannabinoids?
It's able to synthesize 400 slightly different molecules, all of
which act at the cannabinoid receptor... Your brain probably makes a dozen
endogenous opioids. And the opium resin probably contains seven or eight that have
slightly different actions on the body.
Wouldn't you assume that these actions would be modulating or
synergistic?
They might be.
People who've taken marinol say it knocks them on their butt in a
way that marijuana doesn't.
It's pure and it's in high concentration.
And it doesn't have the modulating effects of the other
cannabinoids or trace elements.
But we don't know that there are such things... I just read
a paper today where they found another endogenous cannabinoid in the brain: 2AG. So
the field is just exploding. The question is, what's its normal function?
What's your hyposthesis?
I don't have one yet. I'm not an expert. I hope to
become one. The beauty of this whole thing is not so much that we're going to come
up with another drug to treat pain, but when all is said and done, we're going to know
more about what makes people the way they are. The whole trick is, keep an open
mind. You never know when the next insight's going to come. This new
cannabinoid has very powerful anti-memory action. So you have to ask "Why does
the brain have an anti-memory molecule?"
Obviously there are things too painful to recall.
Or maybe forgetting is part of learning. Maybe its an
endogenous antidepressant. We're going to learn something about memory that we never
would have suspected if it hadn't been for some serendipitous discovery thousands of years
ago that the hemp plant was fun to smoke. What's really exciting to me is that our
knowledge is really exploding; maybe we'll come up with a drug that will improve people's
memory. Maybe it'll be a treatment for Alzheimer's disease. The main thing is,
we've got a lever here for understanding the brain, and only good can come of it as far as
I'm concerned.